Abstract
Background: ChAdOx1 nCoV-19 (AstraZeneca) and Ad26.COV2.S (Janssen Johnson & Johnson) vaccines against COVID-19 have been associated with thrombotic thrombocytopenic reactions referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT) characterized by the presence of platelet-activating, anti-PF4 antibodies. While VITT shares key clinical features with a similar but separate entity, Heparin-induced thrombocytopenia (HIT), there appear to be important differences: 1) VITT patients have extremely high thrombosis rates and are very strongly positive in PF4-polyanion ELISAs, and 2) Many patients with VITT frequently present with refractoriness to therapy or have disease recurrence that suggests distinct antibody characteristics due to a strong autoimmune anti-PF4 response.
Aims: The goal of this study was to characterize anti-PF4 antibodies in VITT.
Methods: Five VITT patients were studied, one after ChAdOx1 nCoV-19 vaccination and four after Ad26.COV2. Reactivity of VITT anti-PF4 antibodies to uncomplexed PF4, PF4-Polyvinyl sulfonate (PVS), and PF4-heparin targets was evaluated, and the platelet-activating ability of these antibodies was examined in the PF4-dependent P-selectin Expression assay (PEA). Anti-PF4 antibodies were isolated from patient blood samples using PF4-treated heparin sepharose beads, and isolated antibodies were subject to mass spectrometric evaluation (Liquid Chromatography Electrospray Ionization Quadrupole time-of-flight mass spectrometry [LC-ESI-QTOF MS]).
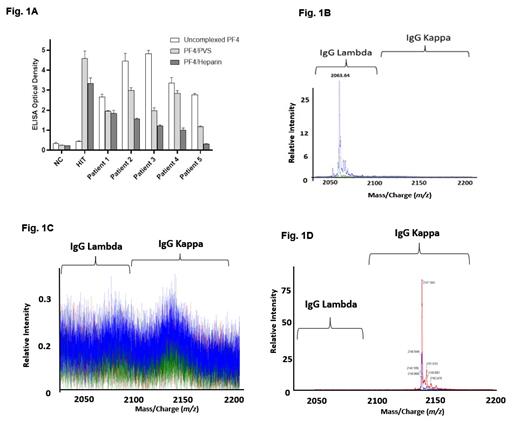
Results: Antibodies from all VITT patients recognized both uncomplexed and complexed PF4 (Fig. 1A). Interestingly, recognition of PF4 by VITT antibodies was lower if PF4 targets were complexed with polyanions, PVS, or heparin (Fig. 1A). These results contrasted with those obtained in a "classical" HIT patient which showed reactivity to PF4/polyanion complexes, but not to uncomplexed PF4 (Fig 1A). All samples activated platelets in the PEA (data not shown). Mass spectrometric evaluation of anti-PF4 antibodies isolated from VITT patients demonstrated monoclonal anti-PF4 antibodies in three patients, and bi- and tri-clonal antibodies in one patient each (a representative monoclonal antibody anti-PF4 antibody is shown in Fig 1B). Consistent with current dogma, polyclonal anti-PF4/polyanion antibodies were seen in "classical" HIT (Fig 1C). Evaluation of anti-PF4 antibodies in spontaneous HIT, a type of autoimmune HIT seen in pro-inflammatory milieus such as orthopedic surgery and infectious prodromes also demonstrated monoclonal anti-PF4 antibodies (Fig 1D). Eluates from control heparin-sepharose beads did not reveal any immunoglobulins (data not shown).
Conclusion: Although development of platelet-activating anti-PF4 antibodies and the thrombotic thrombocytopenia syndrome seen after ChAdOx1 nCoV-19 and Ad26.COV2.S vaccination resembles HIT, these findings demonstrate that clonally restricted anti-PF4 antibodies mediate VITT while polyclonal anti-PF4 antibodies mediate HIT. In addition, we noted clonally-restricted anti-PF4 antibodies in another condition that does not require proximate heparin exposure, spontaneous ("autoimmune") HIT. In VITT, the strong immune response after vaccine administration may result in the activation of a single or few pre-existing anti-PF4 reactive clones, and development of clonally restricted anti-PF4 antibodies with a similar pathophysiology to Spontaneous HIT. It is also likely that high levels of monoclonal/oligoclonal anti-PF4 antibodies cause the severe thrombotic phenotypes seen in VITT and Spontaneous HIT. The high mortality rate and reports of disease refractoriness to therapy in VITT may warrant consideration of additional therapeutic modalities like rituximab and therapeutic plasma exchange in select cases.
Figure Legends: (A): VITT (Patient 1-ChAdOx1 nCoV-19; Patients 2-5, Ad26.COV2.S) patient samples were tested in ELISA against uncomplexed PF4 (white), and PF4 in complex with polyvinyl sulfonate (light grey), or unfractionated heparin (dark gray). (B-D) Mass spectrometric evaluation of anti-PF4 antibodies isolated from VITT (B), HIT (C) and spontaneous HIT patient sera (D). "Relative Intensity" refers to abundance of the Ig light chain relative to the polyclonal background. Numbers above Ig light chain peaks depict mass/charge ratios. NC- Normal control.
Murray: Mayo Clinic: Other: Has received patents for the Mass-Fix technology which has been licensed to the Binding Site with potential royalties.. Padmanabhan: Veralox Therapeutics: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal